- Home
- Life Sciences
- Biotech
Biotech ERP for Dynamics 365
Accelerate innovation and growth with a biotech ERP that supports GMP compliance efforts
Traverse the journey from research to production while enabling compliance with regulatory guidelines. The use of biotech software enables end-to-end visibility in processes and data, making way for data-driven strategic decisions. Optimize warehouse and supply chain and reduce paperwork.
Experience our Biotech ERP system firsthand


Streamline processes for compliance and efficiency with our biotech ERP
Modern biotechnology delivers innovative products and technologies that tackle severe and uncommon illnesses, reduce our carbon footprint, promote sustainable and efficient energy use, and enable safer, cleaner, and more efficient industrial production methods. However, they need to adhere to stringent government standards, keep pace with fast-changing technology, and navigate unpredictable market dynamics.
To enhance your company’s efficiency, you need software that integrates your biotech company’s critical data, from scientific research to financial management seamlessly, all within a single, intuitive platform.
Our biotech ERP helps fine-tune every aspect of your operations, from production to clinical trials. Embedded in Microsoft Dynamics 365 Finance and Supply Chain Management, our solution helps you meet industry standards.
Key Challenges
Regulatory non-compliance
Inconsistent record-keeping and approval processes, inadequate traceability, inefficient change management, and disconnected systems may lead to regulatory non-compliance, resulting in legal action, penalties, and market restrictions.
Extensive paperwork
Inefficient supply chain management
Key Features
Scroll to
Regulatory compliance assistance
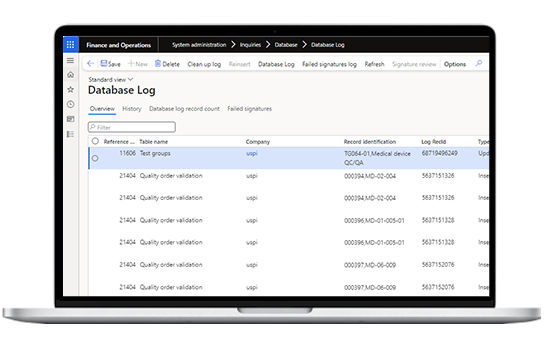
Get compliance support through audit trail implementation, secure batch review and approval processes with electronic signatures to GxP critical fields and tables, near real-time monitoring, and end-to-end traceability.

Supply chain management
Avail all inventory information including location, license plate, item, batch (lot), sub-batch (container), and quantity with a single barcode scan. Maintain an approved vendor, manufacturer, and supplier list. Store inventory as per protocols.

Data management
Manage data and compliance by validating the data through staging tables, setting up access levels to the data, and improving traceability through an audit trail.
Benefits
Integrate with R&D systems and establish seamless data transfer from research to production.
Regulate inventory, procurement, and distribution processes to enable better tracking and control over the supply chain.
Avail seamless data sync, dynamic visualizations, and data security to reduce business risks.
Gain transparent insights into your consumption and movement operations by integrating data from across your supply chain.
Boost customer service by integrating operations and unifying communication.
Get the support to stay compliant with regional and international regulations like FDA 21 CFR Part 11 and EU GMP Annex 11.
Functionality
Compliance Management

Compliance Management
Maintains a comprehensive audit trail for inspections and audits. Facilitates electronic signatures for approvals to help you adhere to industry regulations like FDA 21 CFR Part 11 and EU GMP Annex 11.
Traceability

Traceability
Tracks and traces batch, sub-batch, and container records ensuring traceability compliance.
Inventory Management

Inventory Management
Optimizes inventory levels by real-time tracking. Ensures prescribed storage conditions are maintained to avoid material spoilage.
Supply Chain Management
Supply Chain Management
Manage suppliers, manufacturers, and distribution channels to ensure timely delivery to the market.
Label Printing

Label Printing
Prints shipping and product labels automatically for traceability. Designs printer logic as per user group, zone, warehouse, and locations.
Frequently asked questions
What ERP features are crucial for biotech companies?
Features that support complex manufacturing processes, regulatory compliance, and intricate supply chain dynamics are crucial for biotech companies. Our solution meets standard industry requirements out-of-the-box, eliminating the need for expensive customizations and integrations.
How can a biotech ERP assist me in complying with regulations?
A biotech ERP should have features that provide comprehensive audit trails and sophisticated reporting tools to assist you in complying with critical regulations such as the FDA's 21 CFR Part 11, EMA guidelines, and GMP standards. STAEDEAN Life Sciences Validation Toolkit provides templates and documentation to streamline the system's validation.
What considerations should we have while selecting biotech software?
Assess how well the biotech software handles manufacturing processes specific to biotech industries, such as regulatory compliance, quality control, supply chain management, warehouse management, weighing and dispensing, etc.
How does an ERP support quality control in biotech manufacturing?
The biotech ERP should have robust quality control modules that support in-process inspections, non-conformance tracking, and CAPA (Corrective and Preventive Actions).
Is cloud-based biotech software suitable for biotech organizations?
Cloud-based biotech software offers flexibility, scalability, and remote access, which can benefit organizations with multiple locations or those requiring mobile access. It is safer than on-premises software because cloud providers like Microsoft have stricter security protocols than any biotech company could implement on their own. Cloud-based software security features include access controls, data encryption, and server-based processing.
How can an ERP improve operational efficiency in biotech companies?
Biotech ERP streamlines processes, reduces manual errors, improves inventory management, enhances data visibility, and ultimately leads to cost savings and enhanced productivity.
Resource Center
Scroll to





.png?width=500&height=324&name=LS%20cost%20of%20inefficiencies%20whitepaper%20(1).png)
 Monica Ferraioli
Monica Ferraioli

 Massimo Crudeli
Massimo Crudeli

