- Home
- Life Sciences
- Clinical Supplies
Clinical Supply Chain Software
Enable cost efficiency by optimizing compliance efforts, demand planning, and supply chain
Boost operational efficiency and gain end-to-end visibility with a single clinical supply chain software embedded in Microsoft Dynamics 365 Finance and Supply Chain Management. Save costs through accurate order prediction and streamlined inventory.
Experience our Clinical Supply Chain Software firsthand


Boost productivity and control costs with a single clinical supply chain software
Clinical trials are the foundation of drug development, and crucial for the evolution of medical innovation. Spanning multiple countries and regulatory environments, these complex trials require agile and efficient supply chains. These chains are vital for distributing expensive comparator drugs and sensitive investigative compounds effectively worldwide.
Efficient management of these supplies requires careful supervision of inventory, demand planning, compliance, and logistics. Traditional clinical trial software often falls short of addressing these specialized needs.
Our clinical supply chain software, integrated with Microsoft Dynamics 365 Finance and Supply Chain Management, excels in managing clinical trial supplies. It enhances forecasting, planning, manufacturing, and distribution processes. Adopting our ERP solution gives organizations a competitive edge by efficiently meeting the complex demands of modern clinical trials.
Key Challenges
Lack of supply chain visibility
Lack of supply chain visibility stems from fragmented systems, manual processes, complex regulation, inventory mismanagement, and inadequate data integration resulting in delays, higher costs, and inefficiencies.
Forecasting and planning errors
Regulatory non-compliance
Key Features
Scroll to
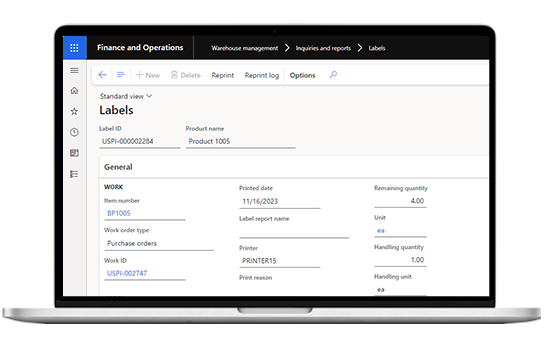
Warehouse management
With clinical trial software, identify material with efficient labeling and printing. Streamline picking, shipment, and distribution with a single scan. Track inventory levels and gain insights for demand planning.
Supply chain management
Manage batch and sub-batch end-to-end with enhanced attributes, and store and distribute as per protocols. Additionally, get assistance in your efforts to comply with FDA 21 CFR Part 11 and EU GMP Annex 11 through electronic signature using our clinical supply chain software.

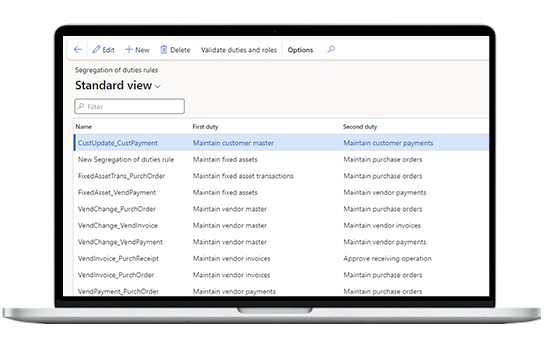
Data security
Handle sensitive data securely by enabling access levels, asset classification, and audit trail. Aid risk management by Segregation of Duties (SoD), Segregation of Privileges, and Segregation of Entry Points to prevent errors, data misuse, and fraud.
Benefits
Connect the entire supply chain to enable end-to-end traceability while complying with regulations. Gain complete visibility to make data-driven decisions.
Use real-time data from our clinical supply chain software to generate predictive insights and forecast demand accurately. Facilitate optimized planning, production, and delivery.
Get compliance assistance through centralized data management, audit trails, and electronic signatures for secure documentation. Receive support to confirm adherence to regulations with near real-time process and quality monitoring.
Boost efficiency and responsiveness through enhanced connectivity. Use predictive insights to preempt issues, ensuring on-time deliveries. Adhere to GMP guidelines, reducing regulatory risks and driving cost-effective, quicker clinical trials.
Track stock and manage expiration dates in real time to optimize inventory levels. This tracking also helps you minimize waste, prevent stockouts, and avoid overstocking, thus saving costs.
Decrease costs by enhancing operational efficiency and reducing inventory overages with our clinical supply management software. Optimized supply chain and logistics reduce the effective cost of clinical trials.
Functionality
Regulatory Compliance Assistance

Regulatory Compliance Assistance
Supports compliance efforts with FDA 21 CFR Part 11 and EU GMP Annex 11 regulations. Generates an end-to-end audit trail for inspections and enables electronic signatures for approval workflow.
Traceability

Traceability
Ensures accurate traceability by tracking batches, sub-batches, and containers.
Inventory Management

Inventory Management
Uses real-time tracking to optimize inventory levels. Ensures that storage conditions are adhered to maintain quality.
Supply Chain Management
Supply Chain Management
Manages suppliers, manufacturers, and logistics to guarantee on-time delivery to the end users.
Label Printing

Label Printing
Prints shipping and product labels automatically for traceability. Creates printer logic based on zones, warehouses, and locations.
Frequently asked questions
Which features are vital in a clinical supplies ERP?
Key features for clinical supply management software include inventory management, supply chain tracking, regulatory compliance assistance, and quality control processes.
How does an ERP enhance compliance with healthcare regulations?
An ERP with comprehensive audit trails and reporting features helps companies comply with FDA, EMA guidelines, and GMP standards.
Can the clinical supply chain ERP handle the complexities of clinical trial supply management?
Yes, our clinical trial software supports the specific needs of clinical trials, such as study design, drug accountability, and distribution management. It is designed to manage complex distribution networks required by modern clinical studies.
How can ERP software improve operational efficiency in clinical supplies management?
A clinical supplies management ERP solution streamlines inventory tracking, automates workflows, provides near real-time data to reduce errors, improves decision-making, and supports compliance with industry regulations and guidelines such as FDA 21 CFR Part 11 and EU GMP Annex 11, which leads to a boost in productivity.
Resource Center
Scroll to





.png?width=500&height=324&name=LS%20cost%20of%20inefficiencies%20whitepaper%20(1).png)
 Massimo Crudeli
Massimo Crudeli